Sample Checklist
The following checklist is a quick reference to ensure your plasmid DNA meets our submission criteria.
Samples that do not meet one or more of the criteria below can be refused, or fail to sequence entirely, so please read the entire document to avoid any issues or delays.
Sample Type: |
Purified Plasmid DNA |
DNA Concentration: |
Normalised to 25* ng/µL |
DNA Volume: |
≥10 µL |
Sample Tubes: |
Strip PCR Tubes |
Sample Labeling: |
4 digit project ID + numbers |
Sample Packaging: |
Rigid, protective packaging |
Sample Info: |
Uploaded to Project Portal |
* 100 ng/µL if measured with spectrophotometer (e.g. NanoDrop)
Sample Type
For PlasmidSeq, we require purified plasmid DNA in an EDTA-free buffer. If you are not sure as to whether your kit’s elution buffer contains EDTA, you can prepare your own by making a solution of Tris HCl pH 8.0 @ 1 mM, or use ultrapure, nuclease-free water.
Sample QC
Miniprep yields can vary dramatically, so it’s important that you quantify your DNA. We also recommend running your samples on a gel, to ensure that a single plasmid is present, there is no significant host contamination, and the plasmid is approximately the right size.
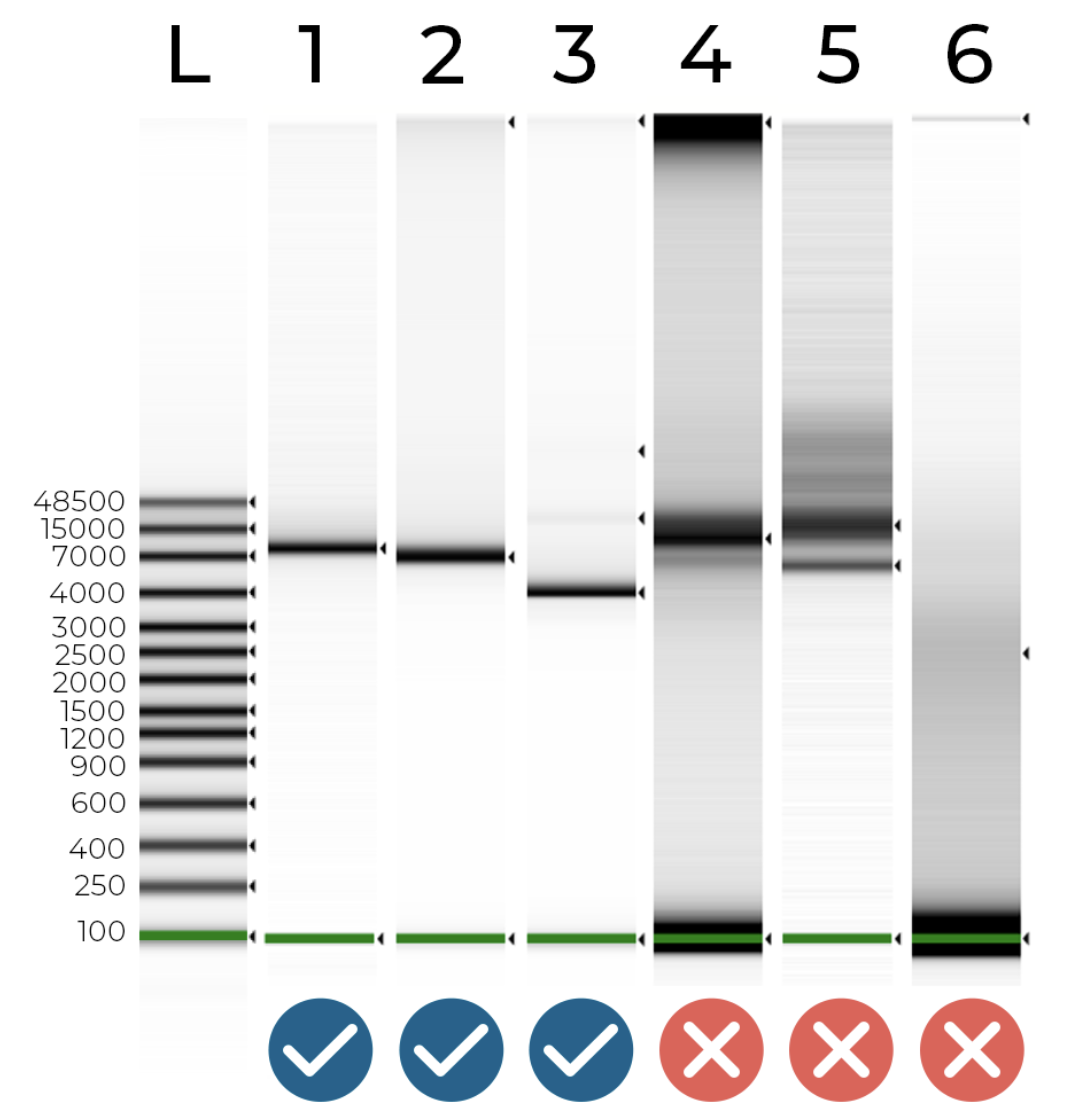
Figure 1 - DNA electrophoresis of 6 plasmids and a DNA ladder. Your samples should look like samples 1 & 2 - a single peak at the expected plasmid size. Sample 3 shows some non-target contamination (this could be a plasmid dimer or a non-target plasmid). Samples 4 and 5 show a significant amount of non-target contamination (e.g. host DNA). Finally, sample 6 - which should be a ~7 kb plasmid - had very little DNA, less than a nanogram! We were able to assemble all of these plasmid samples except sample 6, which just didn’t have enough material to work with.
DNA Concentration
It is important that you normalise your DNA to a concentration of 25 ng/µL (nanograms per microliter).
We’ve noticed that some samples with a much higher concentration are more likely to contain contaminants that inhibit the sequencing process (see note on purity below).
We strongly recommend the use of a fluorometric quantification method that is specific to DNA (e.g. Qubit, Quantus, Quant-iT, Picogreen).
If you do not have access to a fluorometric platform, you can use a spectrophotometric platform (e.g. NanoDrop) to quantify your DNA. However be aware that this modality often overestimates DNA concentration, particularly with plasmid preps.
If you use a spectrophotometer, the minimum concentration we need is 100 ng/µL
DNA Purity
For our library preparation and sequencing to work effectively, the DNA you send us has to be pure. Protein contamination or reagent carryover can inhibit sequencing, so even if you send us a sufficient quantity, an impure sample will produce suboptimal results, and could even fail completely.
You can measure DNA purity using a spectrophotometer (e.g. Nanodrop); pure DNA has a 260/280 absorbance ratio of ~1.8, and a 260/230 ratio of 2.0-2.2.
If your sample is impure, we’d recommend repeating the extraction, or performing a post extraction cleanup (e.g. SPRI-beads).
DNA Volume
We require a minimum volume of 10 µL - though the more you give us, the better!
Sample Tubes
We only accept plasmid samples in PCR strip tubes. Aliquot your samples into a sufficient number of PCR strip tubes. Please ensure you attach the lids securely.
If you don’t have enough samples to fill a whole strip, just leave the remaining tubes empty - you can cut empty tubes off if you want.
If you do not provide your samples in PCR strip tubes, they will not be accepted for sequencing, and will be discarded. This is to ensure we can meet our strict laboratory standards for handling accuracy and maintain fast turnaround times.
Sample Labeling
When you’ve placed your order, you will receive a 4 digit project ID. You need to label the leftmost tube of every strip you’re sending to us with that project ID (see Figure 2).
Then, sequentially number the remaining tubes. This helps us identify any cases where a sample should be present, but no liquid can be observed.
To maintain the highest standards of sample handling and provenance, we cannot accept samples that are incorrectly labeled.
Incorrectly labeled samples will be discarded.
Use a black or blue permanent marker for all labeling, and write clearly and legibly.
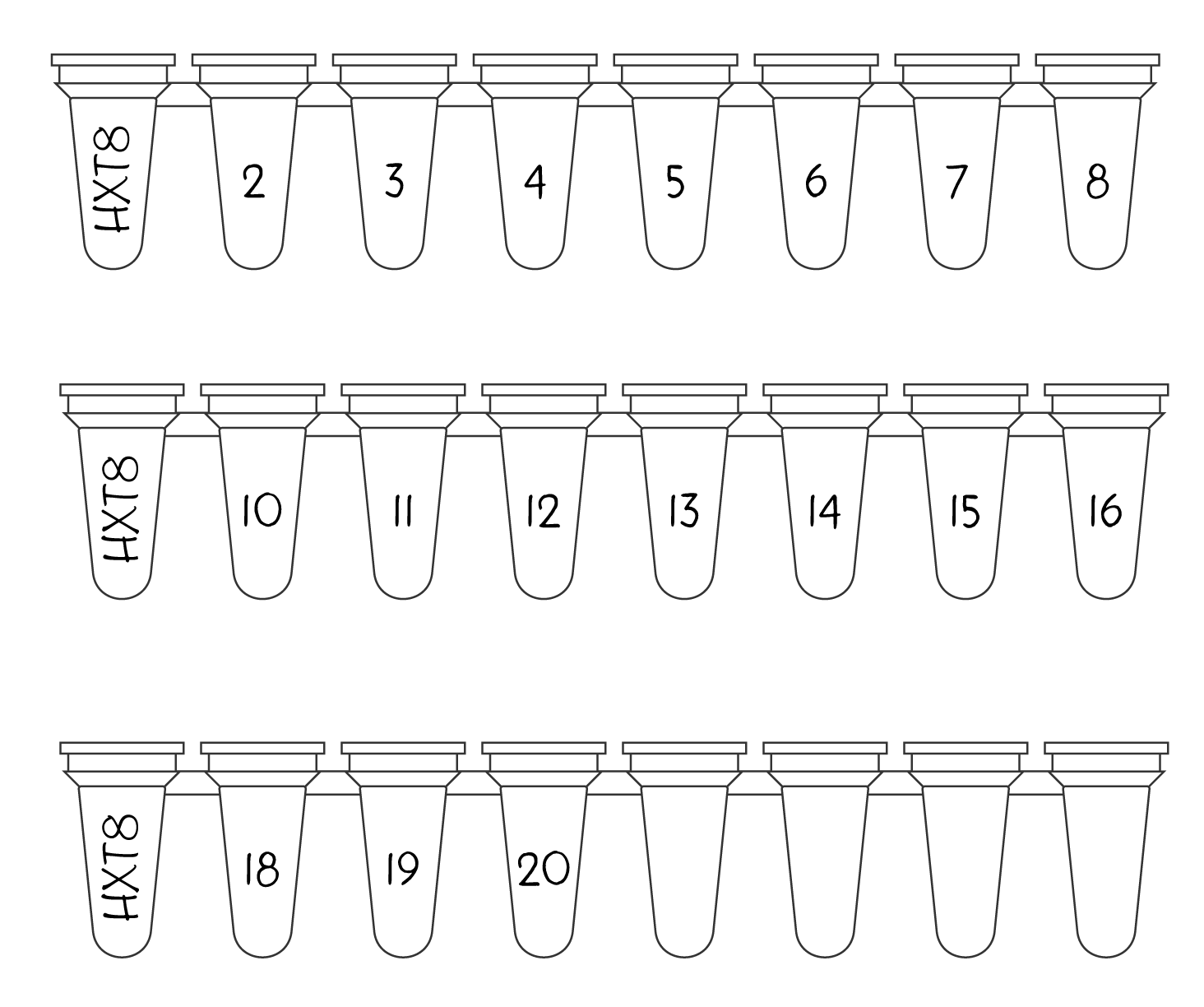
Figure 2 - How to label your PCR strip tubes - For each strip you send us, label the leftmost tube with your 4 digit project ID, and sequentially label each remaining tubes with a number so we know which tubes should contain liquid. If you have extra tubes left on the strip, just leave them blank, or if you prefer, cut them off.
Sample Packaging
It’s important to make sure your samples arrive safely. We’d recommend packing everything in something rigid like a cardboard box with suitable padding - you can also place your strips inside a 50 mL conical tube (i.e. Falcon tube) and slip that inside of a padded envelope or jiffy bag.
Ensure the package is securely sealed, and write your 4 digit project ID on the outside to help us identify it.
Sample Postage
When posting your samples, it’s best to print the postage label to avoid any ambiguity with handwriting. You can get a copy of the label in your project portal.
For all PlasmidSeq orders, our receiving address is:
PlasmidsNG [Order ID]
Units 1-2, The Biohub
Birmingham Research Park
97 Vincent Drive
Birmingham
B15 2SQ
Please ensure you write your order ID on the package to make identification easier.
If you’re shipping internationally, ensure you supply the correct documentation for customs clearance. Samples sent to us hold no commercial value, typically fall under a contents category of ‘Other’, and where required have a harmonised system code (HS Code) of 2934999090.
Dropbox Network
We are developing a dropbox network. If you already have a PlasmidsNG / MicrobesNG dropbox in your institution, you can just drop your labelled samples package in it. Please check your collection date at the dropbox or email us as info@plasmidsng.com.
If your institution does not have one of our dropboxes and you would like to have one you can find out more information here.
Turnaround Time
Our target turnaround time is 1-5 business days, from sample receipt to data delivery.